COVID-19 Antiviral Pills
December 10, 2021
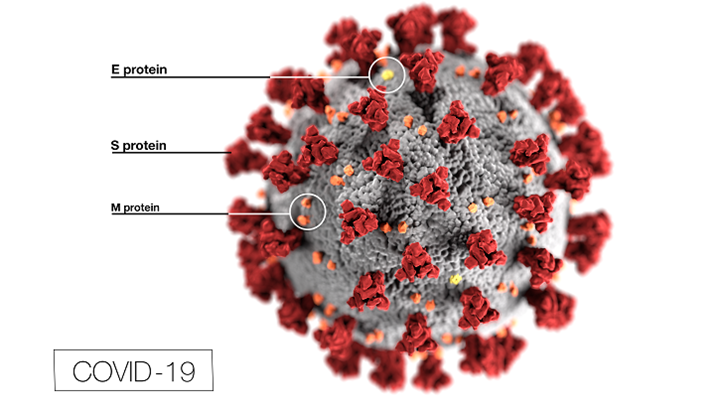
Last week, Molnupirvir, the first oral COVID-19 pill, was approved by the Food and Drug Administration (FDA). This pill, produced by the companies Merck and Ridgeback Biotherapeutics, was approved with a 13-10 vote by the FDA. It comes in an 800-milligram capsule and is to be used every 12 hours. The hope for this pill is that it will minimize the symptoms of those who have contracted COVID, thus reducing hospitalizations. According to CNBC, it is proven that if the pill is taken within five days of the appearance of the first COVID symptom, the severity of the virus is reduced by 30%.
if the pill is taken within five days of the appearance of the first COVID symptom, the severity of the virus is reduced by 30%.
There is also another pill in the works by Pfizer, called Paxlovi d. Although it has not yet been approved by the FDA, it might be by the end of the year. This pill is said to potentially work even better than the Merck pill. Paxlovid has been proven to be 85% effective, which is a very promising percentage. One negative to the Pfizer pill is that it is said to cause problems in patients on other medical drugs. The Pfizer pill must be taken with a drug used for HIV called ritonavir. This too has a downside, as this drug is not able to be used on patients with other medical circumstances as a result of the HIV drug having to be taken as well.
d. Although it has not yet been approved by the FDA, it might be by the end of the year. This pill is said to potentially work even better than the Merck pill. Paxlovid has been proven to be 85% effective, which is a very promising percentage. One negative to the Pfizer pill is that it is said to cause problems in patients on other medical drugs. The Pfizer pill must be taken with a drug used for HIV called ritonavir. This too has a downside, as this drug is not able to be used on patients with other medical circumstances as a result of the HIV drug having to be taken as well.
Overall, these two pills could be the start of more ways to combat COVID. Since vaccines may not be protective against all variants of the virus, these pills might just be the solution to the problem. However, the emergence of the medication may further discourage those who are already hesitant to get the vaccine. These people may believe that if they do get COVID, the pill can save them. Unfortunately, that is not the case. According to The New York Times, Anthony Fauci, the Chief Medical Advisor of the United States, “warned that Americans should not wait to be vaccinated because they believe they can take the pill. While the new medicine may decrease a person’s risk, the best way to be protected is avoiding infection.”