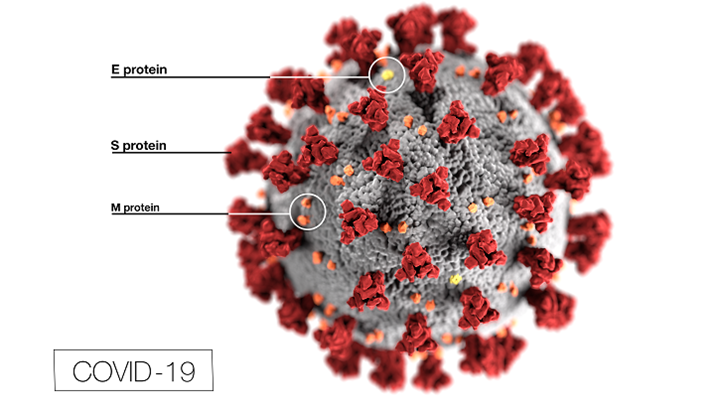
Omicron Variant Increases Demand for Covid Booster Vaccine
December 16, 2021
With the advent of the Omicron COVID variant, health officials are urging all adults to receive the booster shot of the vaccine. After only being available to vaccinated individuals aged 65 and older and high risk adults for several months, the Centers for Disease Control and Prevention is now encouraging all adults aged 18 and older to receive the booster regardless of their health status. Effective November 19, any legal adult who received a Pfizer-BioNTech or a Moderna vaccine is recommended to get the booster shot at least six months after their second dose or two months after their single-dose Johnson & Johnson vaccine.
The Delta variant, which arose over the summer, proved to be more infectious than previous COVID strains and threatened to delay the country’s reopening. Over the summer, the CDC reported that the variant had been detected in over 99% of US cases. An alarming number of those cases were seen in fully vaccinated individuals, prompting the creation of a third reinforcement shot to diminish the risk of contracting the Delta variant. Despite the new vaccine, CDC officials advise the public to “not let their guard down” and to “continue to wear masks regardless of vaccination status.”
Even more recently, the novel Omicron variant has arrived in the United States. Warner Greene, director of the Center for HIV Cure Research at the Gladstone Institutes in San Francisco, explained that “the virus comes with both barrels loaded––high infectivity and potentially the ability for immune evasion. But maybe what it’s lacking is pathogenicity.” Reports from South Africa (where the variant is believed to have originated) indicate that the “Omicron variant of coronavirus is much more contagious than previous variants while causing milder disease.” However, the strain is still in its early stages and definitive data will not be available for weeks.
Pfizer and BioNTech are the first companies to release test results of their booster shot’s efficacy against the Omicron variant. Although their findings are preliminary, when compared, blood samples from those who received two versus three doses of the Pfizer-BioNTech vaccine show that “the third dose of [the Pfizer-BioNTech] vaccine provides neutralizing antibodies against Omicron comparable to those seen against the original coronavirus and other variants after two doses,” as reported by NBC News. These results are promising and have allayed concerns regarding the Delta and Omicron variants.
However, more data is needed to determine if a third dose is truly necessary. Deepta Battacharya, professor of immunology at the University of Arizona, remarked that “while lab data will tell you whether it is capable of escaping the immune response, it won’t really tell you whether we have to do something about it.” Researchers are working to understand how Omicron mutates, but it is still too early to tell how resistant the Omicron variant will be to the third dose of the vaccine. Pfizer and BioNTech’s early results show a notable difference in protection when compared to the two original doses, but is that difference great enough to necessitate the distribution of a third dose?