Ice has long been known for its slippery nature, posing road hazards while also offering the thrill of winter sports such as ice skating or ice hockey. Every winter, we interact with the phenomenon of ice in many different ways and acknowledge its slippery nature, but we may not know exactly why ice has its unique, slippery properties. In this article, you will learn exactly what makes ice slippery and, hopefully, gain an appreciation for this fascinating force of nature.
The slipperiness of ice primarily stems from a thin layer of water molecules on its surface. This partial-liquid layer behaves differently from the solid ice beneath it. This surface layer of ice exhibits remarkable slipperiness due to the unique arrangement and movement of water molecules. These molecules are not as rigidly bound as those in the solid lattice of ice, allowing them to act as a slippery layer. At temperatures close to the melting point of ice, this partial liquid layer becomes even more important. The increased molecular activity at these higher temperatures reduces friction significantly, making the ice even more slippery. However, this effect diminishes at extremely low temperatures, where the ice’s surface layer becomes more similar to solid ice, which on its own is not slippery.
Another contributing factor to ice’s slipperiness is the pressure exerted by objects moving across its surface. When pressure is applied, such as the blades of an ice skate or the tires of a car, it can lower the melting point of the ice near the area where the pressure was applied, creating a thin film of liquid water. This phenomenon, known as pressure melting, further defines this slippery layer of ice, reducing friction and making movement easier. Friction itself plays a two-part role in this scenario. Although friction is usually associated with resistance, on ice, friction can generate heat, which melts a thin layer of the ice surface. This meltwater acts similarly to a lubricant, further adding to the slippery condition.
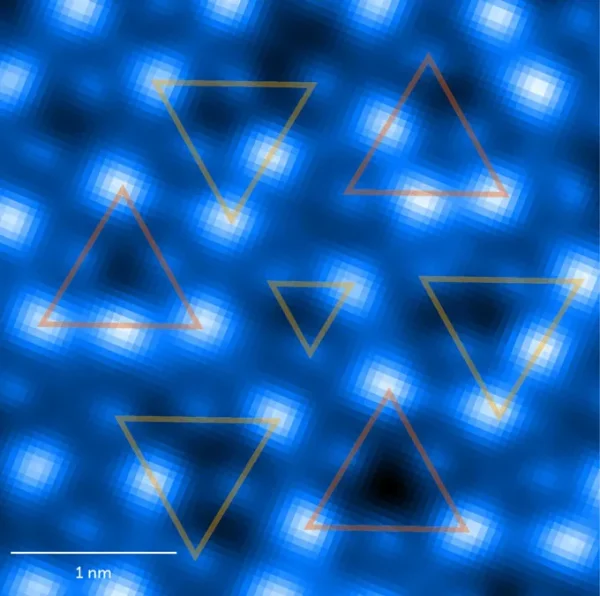
In addition to known explanations, various studies have been conducted for scientists to see the slippery properties of ice for themselves. For example, in a very recent study in May 2024, scientists used atomic force microscopy to “measure the locations of atoms on the surface of ice.” They discovered that at around -150° Celsius, the surface consists of two types of ice, according to physicist Ying Jiang of Peking University and colleagues, who reported to Nature on May 22.
Ice can exist in many different forms, depending on the arrangement of its molecules. Connected to this point, the slipperiness property of the ice also has much to do with how the atoms and molecules are arranged in the ice. When the study was conducted under normal conditions, water molecules formed layers of hexagons, creating hexagonal ice known as ice Ih, which was the focus of Jiang et al.’s study. However, in addition to these hexagonal ice layers, the atomic force microscope also revealed images that indicated that other regions of the surface consisted of ice lc, where “hexagons in each layer are shifted to create a structure similar to the arrangement of carbon atoms in diamond.”
At the borders of the two ice types, researchers found structural defects caused by the misalignment of their patterns. When the temperature was slightly increased, these “disordered” areas expanded along the ice structure. As the temperature was raised even further, these disordered structures became even more prominent. Eventually, at even higher temperatures, the team experimentally concluded that “the disorder would expand to cover the entire surface of the ice, giving ice its full liquidlike patina.”
The slipperiness of ice is indeed a unique phenomenon worth diving further into. It’s one thing to understand the simple properties of ice, but knowing the science behind why ice is known for those properties can lead us to better understand nature’s various phenomena and can also give us an even greater appreciation for them.