The Nobel Prize in Chemistry 2017
November 18, 2017
Many scientific discoveries are developed every year, so how do you decide which one has been or will be the most vital? This is where the Nobel Prize comes into play. The Nobel Prize in Chemistry is an honorary award that has been awarded annually by the Royal Swedish Academy of Sciences. This award is given to chemists who have made a groundbreaking discovery that has been or will be extremely useful in the general field of science.

In 2017 this honor was awarded to Joachim Frank, Richard Henderson, and Jacques Dubochet. Joachim Frank is an American biophysicist at Columbia University, New York City. Richard Henderson is a Scottish molecular biologist and biophysicist. Jacques Dubochet is a respected professor of biophysics at the University of Lausanne in Switzerland.
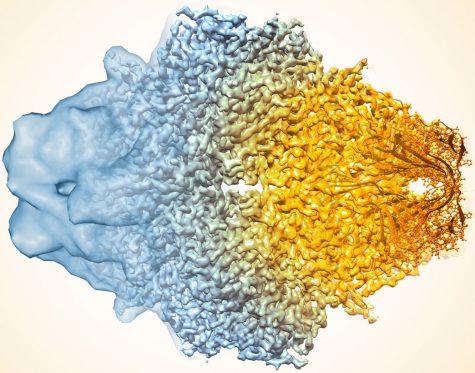
These three chemists won the Nobel Prize for creating cryo-electron microscopy that can determine high-resolution structures of bio molecules in solution. This technique enables scientists to take unprecedented views of important bio molecules.
In cryo-electron microscopy (cryo-EM), a biomolecular sample is frozen, usually with liquid ethane and then a beam of electrons is sent through. The electrons are deflected by the material in a certain unique way that enables scientists to understand the structure of the biomolecule. One of the major problems with the electron beams is that they damage the biomolecules, but freezing them allows the biomolecules to avoid electron damage. They also stay hydrated by being kept in a vacuum chamber located in an electron microscope.
Biomolecular structures observed using cryo-EM is incredibly vital to the understanding of life chemistry. According to the magazine C&EN, there are many ways that cryo-EM can be used to boost our understanding of life chemistry, one of which is that it “can help scientists develop drugs by elucidating the way bioactive agents react with biomolecules.” The technique of cryo-EM will help scientists save lives.
X-ray crystallography and nuclear magnetic resonance spectroscopy are traditional methods to understand the structure of biomolecules, but cryo-EM is much more efficient. Cryo-EM can create quick and effective visuals of molecules other methods could not do. Also cryo-EM avoids the creation of crystals in the biomolecules, which creates interference with identifying structures of biomolecules.
Dr. Bingxin Shen was a former member in Joachim Frank’s lab, and currently is an assistant professor at Temple University, Philadelphia. Dr. Shen believes that with the method of Cryo-EM many medical issues can be solved. “We can react faster with any new breakout disease to get the structures of the related new virus and find a general cure. We could design personalized medicine for human diseases based on everyone’s different protein structure,” she said. She also believes that with the advancement of cryo-EM, the age of science will evolve even further. “As we got faster and faster computing power, we could get closer to AI. As we get finer and finer views of living cells, we will get closer to the secret of life!”
Dr. Oliver B. Clark and Joachim Frank have collaborated on many papers before. When asked about whether he thought that cryo-EM would further evolve the age of science, he said, “Well, it certainly adds another level to our understanding of the structures that underpin biology, in addition to allowing us to characterize static structures in isolation, cryoEM gives us both context and dynamics. Context, in that by using tomography we can now characterize the structure of proteins in their native cellular environment, and dynamics, in that cryo-EM allows us to obtain not just a single structure for every sample but multiple, so if we have a molecular machine that is opening and closing, we can obtain the structures of both the open, the closed, and potentially intermediate states from the same sample. This allows us to better understand how the machine works, and how it operates in the context of a cell.”
This work in advancement of cryo-electron microscopy is definitely deserving of the Nobel Prize in Chemistry.